The Biocompatibility of a New Type of 45S5 Bioactive Graft in a Sheep Model: A Pilot Study Erhan Okay 1, Ali Can Ozarslan 2, Özgür Başal 3, Hüseyin Cakıroglu 4, Sevil Yucel 2, Korhan Özkan 5, Mahmut Nedim Doral 6 7

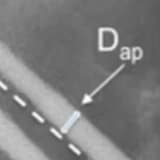
Background Bone is a dramatically regenerating tissue with the ability to heal after trauma, although intensive surgical management is required to treat considerable damage. In this study, 45S5 bioactive grafts were prepared through the melt-quenched method in compliance with the guidelines on medical product requirements (MDD regulations; 93/42/EEC Annex-II section 3&4 and ISO standardizations; ISO 13485:2016) for bone repair and regeneration. Methodology After preparing the graft/scaffold, it was evaluated for biocompatibility according to the principles of "lSO 10993-6 2015 Biological evaluation of medical devices: Tests for local effects after implantation, Annex D 'Test method for implantation in bone,'" "lSO 10993-2:2005 Biological evaluation of medical devices: Animal welfare requirements," and "lSO 10993-12 2012 Biological evaluation of medical devices sample preparation rules and standards." Defects were created on the tibia of the right hind leg. The defects were filled with 3-mm bioactive granules, and a cylindrical polypropylene biocompatible material was used as a negative control. After 120 days, the sheep were sacrificed, and the tibia were analyzed. Results The results demonstrated the safety of 45S5 bioactive grafts. Histological evaluation showed no signs of pathological changes around the implant area. Hematoxylin and eosin sections demonstrated the presence of a few multinucleated giant cells, macrophages, and non-irritant mild fibrotic changes on the surface of the biomaterial. Conclusions 45S5 bioactive glass was found to be biocompatible in a sheep model, demonstrating its capacity to promote bone consolidation while also justifying its further preclinical application as a bone-bonded material owing to the layer formation of the growing bone mineral.
Keywords: bioactive material; biocompatibility; experimental animal model; experimental sheep surgery; preclinical research.